Context: Thrombocytopenia (<50 x109/L) is seen in 10-30% of CMML and is associated with worse prognosis (Itzykson JCO 2013, Patnaik Leukemia 2013). It may have a central and/or a peripheral mechanism. Eltrombopag (ELT), an oral TPO analog, has shown efficacy in low risk MDS with no increased risk of disease progression (Oliva Lancet Hematol 2017) but data is limited in CMML and disease progression has been seen in patients with high risk features (Ramadan Clin Lymphoma Myeloma Leuk 2016). We report the final results of a multicenter phase 2 study investigating ELT efficacy, toxicity and biomarkers in CMML pts with platelets (PLT) <50 x109/L (NCT02323178).
Methods: In this a phase I/II, open-label, single arm, multicenter study, key inclusion criteria were: WHO 2008 defined HMA-naive CMML, PLT < 50 x109/L, marrow blasts ≤ 5%, IPSS low/int-1 in MD-CMML, and in MP-CMML no or only 1 severity criteria (Hb < 10 g/dL, ANC > 16 x109/L, abnormal karyotype, extramedullary disease), spleen size < 16 cm.
ELT was started at 100mg/d (amended to 50 mg/d) with escalating dose up to 300mg/d for at least 12 weeks. Primary endpoint was Platelet Response (HI-P) at 12 weeks, according to IWG 2006 criteria. Responders could continue ELT until protocol-defined progression, loss of response or toxicity.
Results: Between August 2014 and September 2018, 30 pts (median age 77.5 years; M/F 22/8) including 21 MD-CMML and 9 MP-CMML were enrolled. 19 pts had CMML-0 and 11 pts CMML-1 with median PLT count of 32 x109/L (IQR 21-43 x109/L). 12 pts were PLT transfusion dependent (PLT-TD). In the 28 pts sequenced, TET2 mutation (mut) was found in 26 pts, RUNX1mut in 16 pts, SRSF2mut in 11 pts, ASXL1mut in 9 pts, signaling mut in 10 pts and PHF6mut in 5 pts.
Median ELT dose at 12 weeks was 150 mg/d (IQR 100-262.5 mg/d). At 12 weeks, 14 pts (46.7%) achieved HI-P (95%CI 28 ; 66%) including 10 MD-CMML and 4 MP-CMML irrespective of PLT-TD status (p=0.46). Responders and non-responders mutational profile was comparable except that none of the 5 PHF6mut pts responded (p=0.06). Responders received ELT for a median of 33 weeks (IQR 17.3-49.5 weeks) with one responder still on therapy at 24 months. Median duration of response was 3.4 months (95%CI: 1.7-11.6 months). Loss of response were due to PLT decrease (11 pts) or transfusion (5 pts) and disease progression (3 pts).
At 12 weeks, bleeding symptoms (all grades) were present in 3 (38%) non-responders and 4 (29%) responders.
Clinical and biological grade≥ 3 adverse events (AE) were reported in 15 pts each. Before 12 weeks, 24 clinical and 16 biological AE occurred in 7 and 10 pts resp. Toxicity was cardiovascular, pulmonary or gastro intestinal in 2 pts each resp., hepatic and musculo-skeletal in 1 pt each and others in 3 pts. Biological toxicity was hepatic in 4 pts, electrolytic in 4 pts and others in 3 pts. Five pts discontinued ELT due to persistent drug related toxicity. No therapy-related deaths were reported.
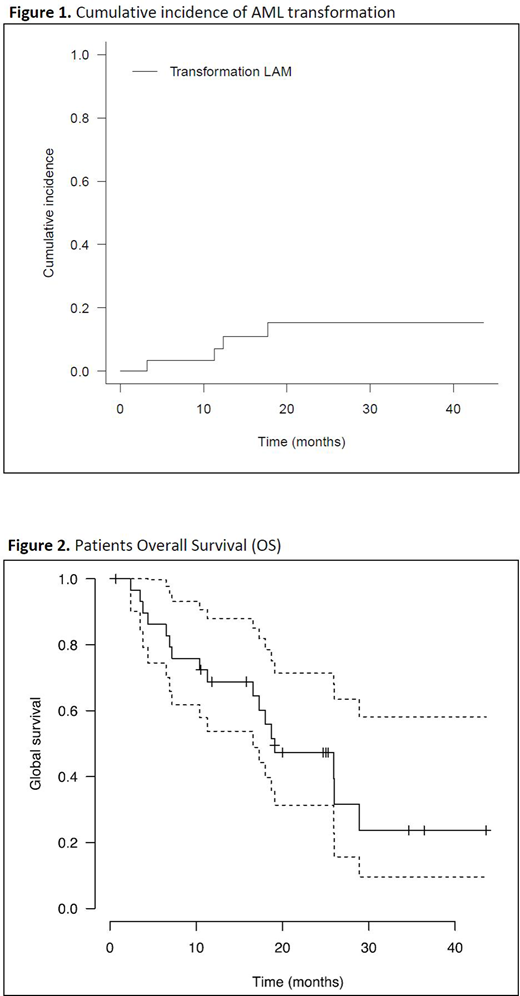
With a median follow-up of 17 months, 14 pts progressed (including 4 AML transformations) and 17 died. The12-month cumulative incidence of AML was 7% (95%CI: 1-21%). No factors were associated with risk of transformation in univariate analysis (neither WBC nor molecular mutational profile). Two-years OS and PFS were 47% (95%CI: 31-71%) and 28% (95%CI: 15-52%) respectively. Splice mutations were associated with better PFS in univariate analysis (HR=0.29, (95%CI: 0.11-0.76%); p=0.012). In the 21 CMML pts with PLT < 50 x109/L and ≤5% BM blasts from our previous CMML cohort (Itzykson JCO 2013) who were not exposed to ELT, the 12-month estimates cumulative incidence of AML was 10% (95%CI: 0-23%). Comparison with a larger historical cohort not exposed to ELT is ongoing.
Conclusion: In CMML patients with severe thrombocytopenia and no marrow blast excess, treatment with ELT is safe and induces frequent but mostly transient responses without increasing the risk of CMML progression. ELT could thus help manage a situation at risk of bleeding such as a scheduled surgical procedure. Phase III studies may be useful to confirm the role of ELT in CMML patients with thrombocytopenia.
Rabian:Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria. Thepot:astellas: Honoraria; novartis: Honoraria; sanofi: Honoraria; celgene: Honoraria. Braun:Daiichi Sankyo: Honoraria; Servier: Research Funding. Ades:jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; novartis: Research Funding; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Celgene/BMS: Research Funding. Solary:Janssen: Research Funding. Itzykson:Oncoethix (now Merck): Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka Pharma: Membership on an entity's Board of Directors or advisory committees; BMS (Celgene): Honoraria; Janssen: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Sanofi: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria; Abbvie: Honoraria; Stemline: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal